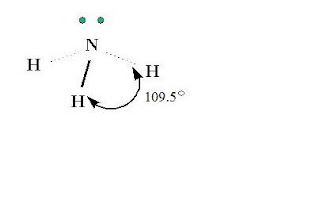
Ammonia is a molecule, which is a combination of nitrogen and hydrogen. Ammonia has the formula of NH3. Nitrogen is the central atom and has five valence electrons. Hydrogen has one valence electron. Each of the three hydrogen atoms share its electrons with nitrogen to form a covalent bond. Nitrogen has two left over electrons that do not bond.
3-D Structure of Ammonia
- shape: AX3E (triangular pyramid)
- angles: 109.5 degrees

Image Sources:
http://newenergyandfuel.com/http:/newenergyandfuel/com/2008/09/30/a-fast-way-out-of-using-gasoline/nh3/

I love the colors and how organized the page is. I think you did a great job. It's very eye-catching.
ReplyDeleteThe molecule is very accurate. I like the use of angle measures, as well. The bonds are also shown well. In your other pictures, you also show the molecule very well.
ReplyDeleteI love the background pattern and colors. The font color, background and even molecule colors all go together to create a visually appealing color scheme. It is well organized, well spaced and very neat. Great job!
ReplyDeleteThe 3D molecule is helpful as it shows the accurate shape of the actual molecule. The other diagram is also very informative as it not only shows the elements that bond to make the molecule but also the forces between them. Together, the two pictures give accurate depictions of the molecule from different perspectives.
ReplyDelete